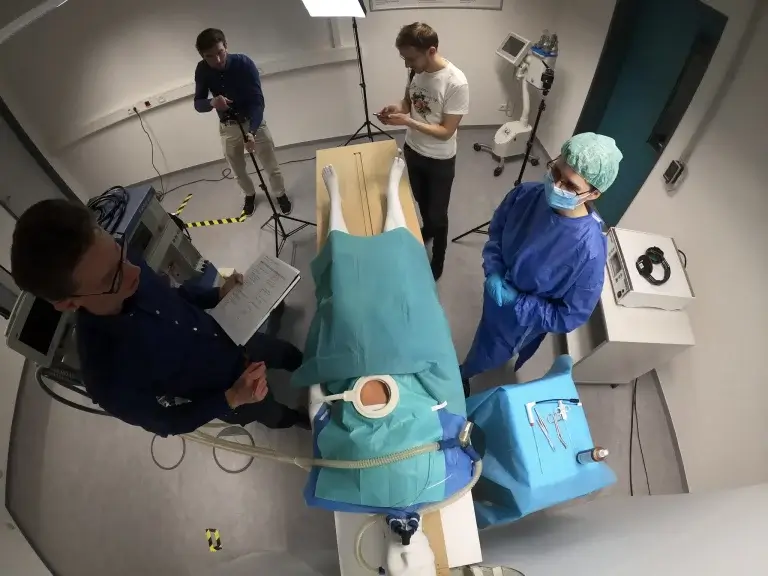
The world of medical technology never stands still, and at the heart of this relentless development is the indispensable need to continuously improve the safety and effectiveness of medical devices. A shining example of this progress is the usability lab designed by USE-Ing. at the STIMULATE research campus. This initiative is not only a testament to technological innovation but also to the fruitful collaboration between renowned institutions and companies.
Innovative collaboration for forward-looking solutions
The creation of the usability laboratory with a focus on iMRI (interventional magnetic resonance imaging) is the result of the joint efforts of Otto von Guericke University, USE-Ing. GmbH and Hannover Medical School. This specialized environment was developed with the aim of addressing the unique challenges and requirements of interventional radiology. Equipped with a simulated interventional room, an interactive MRI model, a control room and an observation room, the lab provides a comprehensive platform for studying clinical workflows, identifying challenges and improving the usability of medical equipment.
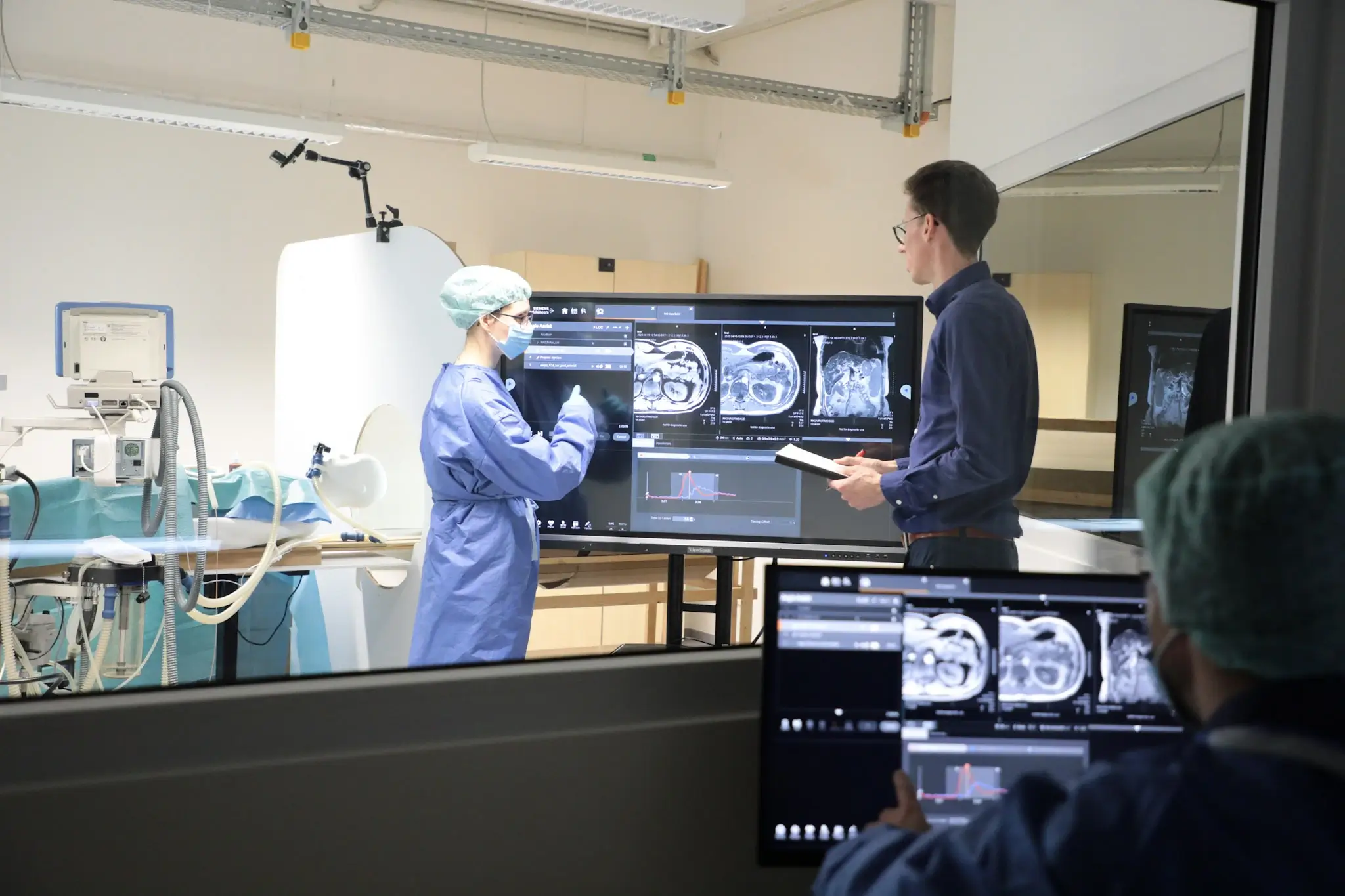
Success through partnership & technology
A recent interim evaluation as part of the BMBF-funded STIMULATE research project has proven the efficiency of the laboratory by demonstrating its ability to simulate MRI-assisted liver biopsy procedures as an exemplary use case. This success was made possible by the collaboration with renowned partners such as the University Medical Center Magdeburg, BEC GmbH and IGEA S.p.A.. Their expertise and dedication not only made this development possible, but also made it extraordinarily fruitful.
Our role as USE-Ing. & the importance of usability tests
We at USE-Ing. see ourselves as a key player in this project, as we were able to demonstrate our expertise in the field of human factors and usability engineering. The combination of quantitative measurement methods such as eye-tracking and motion-tracking, paired with classic usability test methods, significantly shaped the evaluation objective. The innovative character was additionally reinforced by the collaborative evaluation concept, whose true added value lies in the mapping of real workflows. Several test subjects, a radiologist and a radiology assistant, went through the usability test and their respective collaborative tasks in parallel. This orientation has a massive influence on the test design and the work of moderators and note-takers.
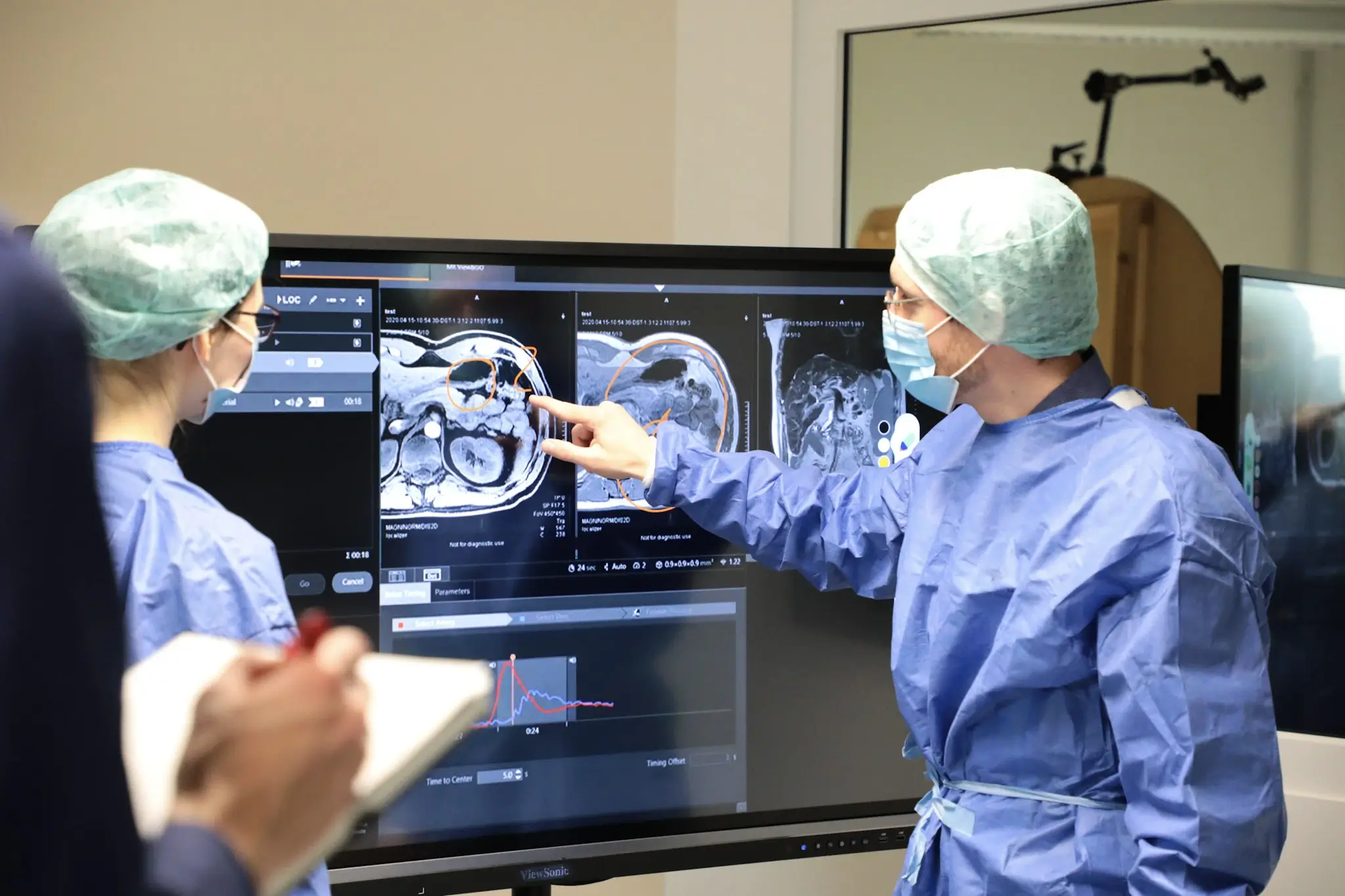
By providing comprehensive services ranging from context of use analysis, usage risk analysis, user interface design and user interface evaluations, we are compliant with relevant standards such as IEC 62366 and ISO 14971. These services are critical to ensuring the safety and effectiveness of medical devices, minimizing risks and ultimately improving patient care.
Future prospects & the potential for further innovations
The usability laboratory at the STIMULATE research campus and the involvement of USE-Ing. impressively demonstrate how research, development and practical application can go hand in hand. This project serves as a model for future research and development projects and offers a glimpse of what can be achieved through collaborative usability tests in the future.
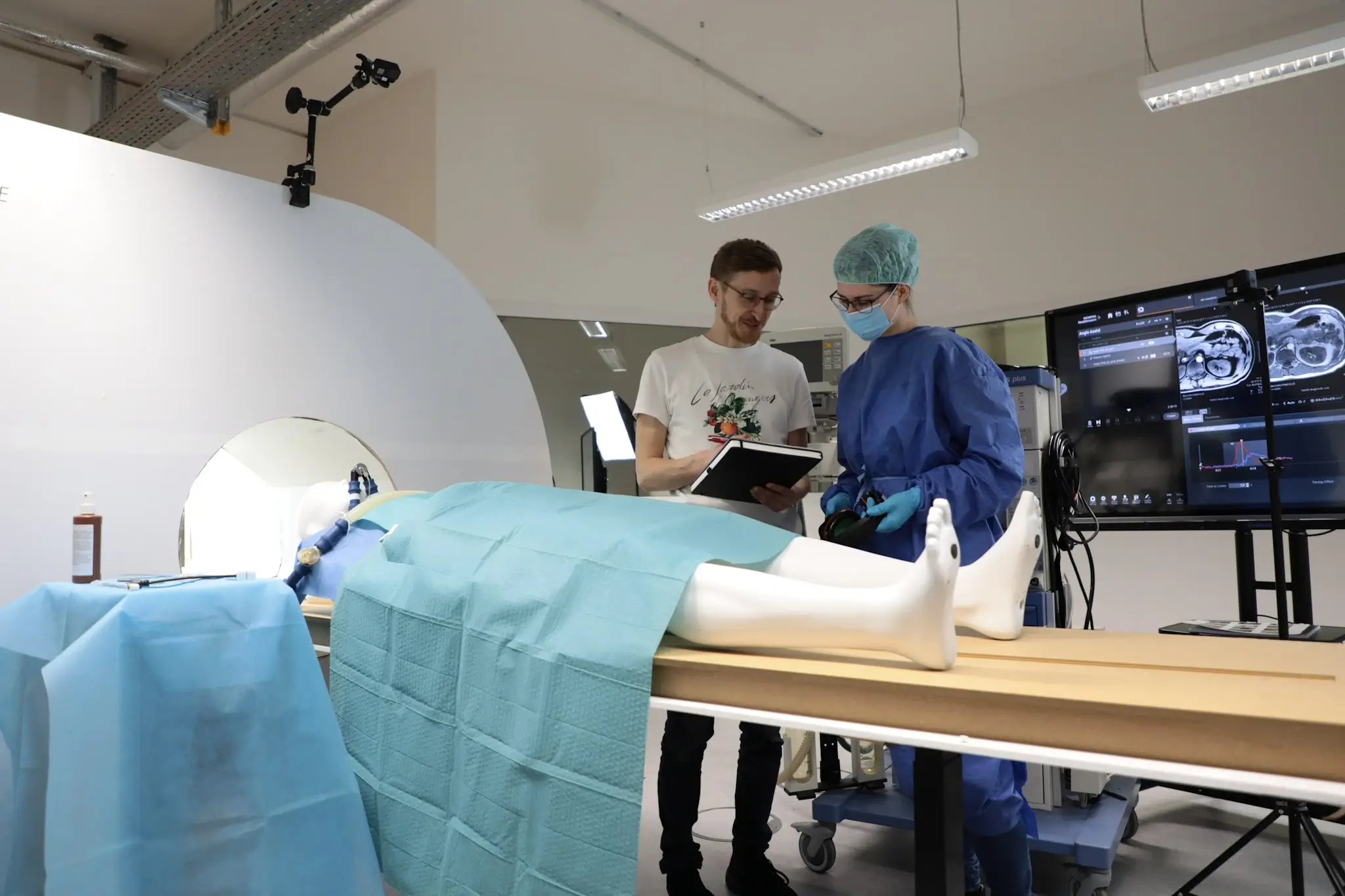
The successful implementation of this milestone at the STIMULATE research campus underlines the importance of collaboration between science, industry and medical institutions. It is living proof that by combining expertise, commitment and innovative technologies, the boundaries of what is possible in medical technology can be constantly expanded. We would like to thank all our partners, especially VDI Technologiezentrum GmbH for their exemplary management, for their contribution to this success and look forward to continuing to work together to set new standards and drive innovation and report on this in the near future.
STANDARDS & REFERENCES
- Show Case Video: Collaborative Usability Testing
- IEC62366-1: Teil 1: Anwendung der Gebrauchstauglichkeit auf Medizinprodukte (IEC 62366-1:2015 + COR1:2016 + A1:2020); Deutsche Fassung EN 62366-1:2015 + AC:2015 + A1:2020
- DIN EN ISO14971: Medizinprodukte – Anwendung des Risikomanagements auf Medizinprodukte (ISO 14971:2019); Deutsche Fassung EN ISO 14971:2019 + A11:2021